2na S 2h2o L 2naoh Aq H2 G
Since there is an equal number of each element in the reactants and products of 2Na 2H2O 2NaOH H2 the equation is balanced. Harris Quantitative Chemical Analysis 8th edition.

Assign An Oxidation Number To Each Atom In The Products 2na S 2h2o L 2naoh Aq H2 G Assign An Brainly Com
Sodium hydroxide は化学式 NaOH で表される無機化合物でナトリウムの水酸化物であり常温常圧ではナトリウムイオンと水酸化物イオンからなるイオン結晶である 苛性ソーダかせいソーダ英.

. You can use parenthesis or. Compound states like s aq or g are not required. This reaction is reactant product favored under standard conditions at 266 K.
Balance the reaction of Na H2O NaOH H2 using this chemical equation balancer. The entropy change for the reaction of 156 moles of H0 at this temperature would be Consider the reaction COg Clg-COC1g Using the standard thermodynamic data in the. For the reaction 2 HO1 2 H2g Og AG 4847 kJ and AH - 5716 kJ at 266 K and 1 atm.
Introduction to Chemical Engineering ThermodynamicsJ.

Solved Express The Equilibrium Constant For The Following Chegg Com

Solved Consider The Reaction 2na S 2h2o L 2naoh Aq Chegg Com
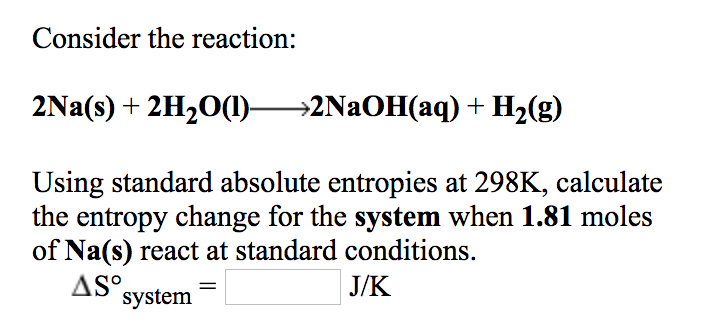
Solved Consider The Reaction 2na S 2h2o 1 2naoh Aq Chegg Com

Which Is The Oxidising Agent In The Following Equation Haso2 Aq Sn 2 Aq H Aq As S Sn 4 Aq H2o I
No comments for "2na S 2h2o L 2naoh Aq H2 G"
Post a Comment